Services for your needs
DelSiTech provides drug delivery services in the pharma and biotech industry. We work with our partners to get the most out of their innovations and turn new product ideas into novel and commercially viable therapeutic drug products.
Typically, a new project starts with a feasibility study to demonstrate that silica drug delivery technology is compatible with your active molecule or active component (e.g. viral vector). This includes development and manufacturing of preliminary silica-based formulations and testing of the drug release profile in in vitro dissolution studies, according to the targets that you have set for the product. In addition, we can manufacture and supply, at non-GLP/GMP quality level, test products for preliminary animal studies to investigate the in vivo pharmacokinetics and/or efficacy of the controlled release product.
Upon completion of successful feasibility studies, DelSiTech can continue to work with you, by either transferring the technology to your site or DelSiTech taking the main responsibility for additional studies on your behalf. DelSiTech provides R&D services up to clinical phase 2 studies, including preclinical development services (non-clinical toxicity, pharmacokinetics, animal efficacy models), pharmaceutical studies (formulation development, analytical development, stability studies, manufacture of clinical trial material) and early clinical development (phase 1-2 clinical studies in healthy volunteers and patients).
Small molecule drug delivery
Versatile technology for small molecule drug delivery
Biodegradable silica-based technology offers multiple options for small molecule drug delivery needs. We can tailor make the best solution for your molecule whether it is a new chemical entity or an already known drug.
We have developed dozens of silica-based formulations to various sized small molecules, ranging from practically water-insoluble drugs to highly-water soluble hydrophilic compounds. The release time can vary from a few days to several months. Also heat and/or organic solvent sensitive molecules can be encapsulated as the manufacturing can be made at cold temperature in aqueous solutions.
Delivery solutions for macromolecules
Controlled release of biological macromolecules
With DelSiTech’s silica matrix you can administer peptides, proteins like antibodies, RNA, DNA and complex carbohydrates in a controlled fashion.
DelSiTechTMSilica Matrix is the method of choice for controlled drug delivery of biological macromolecules. We have successfully developed numerous formulations for peptide and protein drug delivery. The protein release rate is strictly controlled by silica biodegradation and the biological activity of encapsulated proteins is well preserved. Also, complex carbohydrates such as heparins can be administered with our technology. In addition, DelSiTechTMSilica Matrix is well suited for drug delivery of therapeutic RNA molecules (e.g. siRNA) and DNA plasmids where conventional administration methods often fail.
Virus vector delivery
First-in-class controlled delivery and thermostability of viral vectors
It is possible to administer viral vectors (e.g. in gene therapy, vaccination) in a controlled fashion, for longer time periods. Silica encapsulation also offers other unforeseen benefits to virus-based therapeutic products.
DelSiTech is the technology pioneer in encapsulation of virus particles in biodegradable silica. DelSiTechTMSilica Matrix contains a large amount of water which ensures the preservation of the biological activity of virus vectors. In addition, all manufacturing steps can be made at cold temperature in aqueous solutions. We have successfully developed products that release functional virus particles for up to one month. The dosage form can be an injectable depot or an implant.
A unique feature of silica encapsulation of viruses is the possibility to store products even at room temperature. We have shown that virus particles stay functional in DelSiTechTMSilica Matrix for up to 18 months when kept at room temperature or at 4 °C. This is of course a great benefit when planning the logistics and storage of viral vector products as most gene therapy products today need to be stored at -80 °C.

Ocular drug delivery
Unique drug delivery platform for ophthalmology products
Drug treatment for ocular diseases is challenging due to the complex and protected structure of the eye, which prevents the penetration of active compounds at the site of action. Virtually all drugs targeting back of the eye will need a controlled delivery system to enable a long-lasting drug effect inside the eye and avoid repeated intravitreal injections.
Although topical administration of eye drops is an easy and non-invasive method of ocular drug therapy, it suffers from low and easily saturated dose, poor bioavailability due to rapid drainage, and low patient compliance. Invasive systems such as intravitreal (IVT) injections are used to treat the back of the eye. However, IVT injections cannot be given repeatedly in a short period of time because they are associated with potential severe side effects, such as increased pressure in the eye or infection.
DelSiTech has developed two types of Silica Matrix-based formulation platforms that can be used in ophthalmic drug delivery.
Ophthalmic Silica Composite Eye Drops:
- One drop applied topically in conjunctiva cul-de-sac once daily
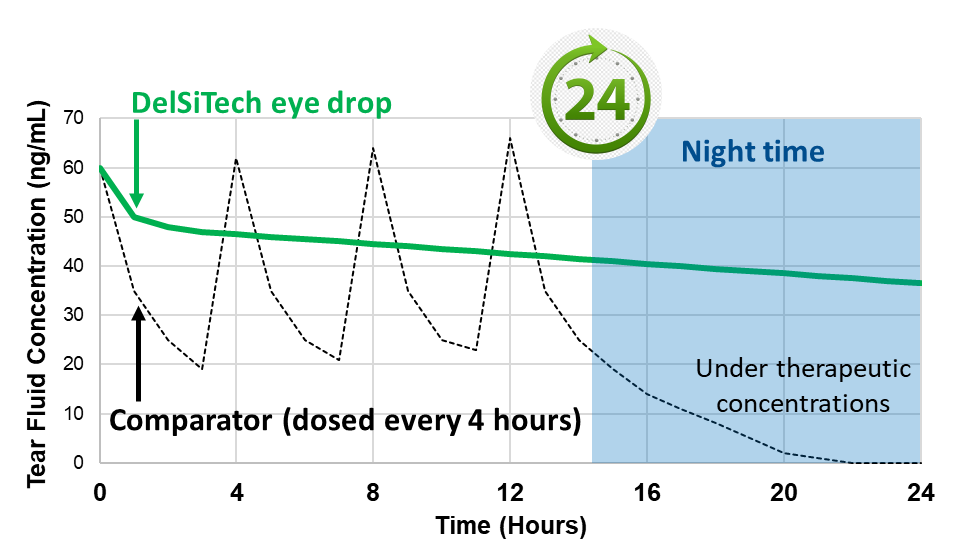
- Sustained drug concentration in the tear fluid over 24 hours
- Steady state dosing
- No variation of drug concentration unlike repeated dosing
- Therapeutic doses even at night
- Well tolerated and comfortable to apply and use
Injectable Silica Composite for Intravitreal Delivery:
- High drug loading of small molecules or large biologics
- Injections with a needle of 30 gauge
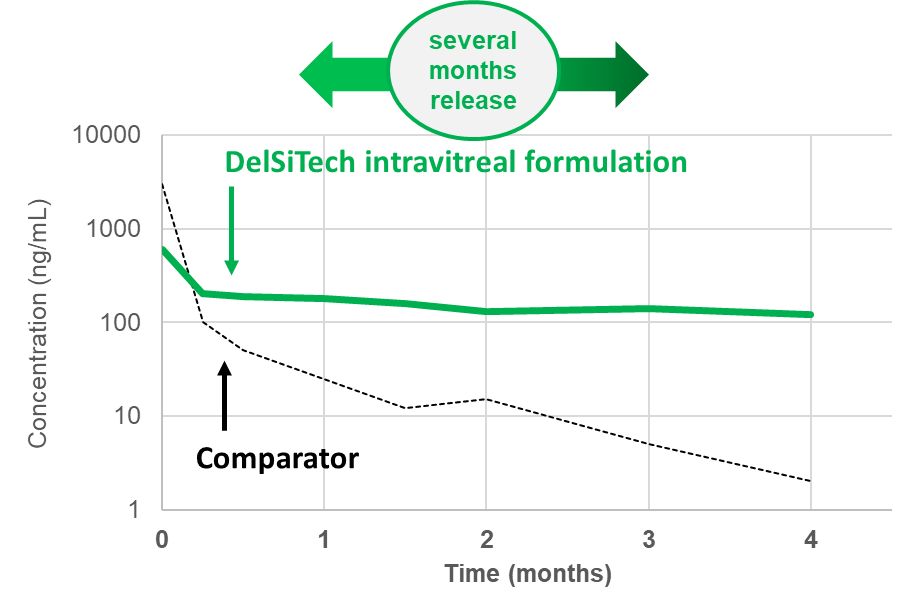
- Stable drug release form several months up to a year
- Zero order release
- low burst to burst-free release
- Well tolerated
Manufacturing service
Tailor-made spray drying manufacturing up to pilot scale
DelSiTech is a manufacturing specialist in spray drying. Our manufacturing service covers all your needs from preparation of small non-GLP test material batches for early pharmacokinetic and efficacy studies up to clinical products manufactured under cGMP
DelSiTech provides small scale microparticle manufacture by spray drying in its own laboratory using Büchi benchtop instruments. We can also perform spray drying in an isolator for more hazardous substances. With this system, we can produce batches from 0.1 g to 100 g, which is typically enough for early development purposes and even for early GLP toxicity studies where there is no requirement for test items manufactured under cGMP. To produce larger batches of microparticles under cGMP, we collaborate with our manufacturing partner to whom we have transferred our technology.
Analytical service
State-of- the-art analytical support for early stage development
DelSiTech provides high quality analytical support to its partners. With highly skilled and experienced scientists and state-of- the-art instrumentation, DelSiTech can deliver efficient and fast analysis service especially for early stage development where no GLP/GMP compliance is needed. Our services include compound and formulation analyses, preliminary stability studies, degradation studies, particle size analyses, rheological property analyses, dissolution testing and method development. We also offer tailor made analytical services for biological macromolecules such as proteins and peptides. Our service model is very flexible, and we are able to accommodate also the needs of small companies and academic groups with limited budgets.
Preclinical service
Fast transition from formulation development to early preclinical studies
DelSiTech can coordinate all of the necessary preclinical studies for a project.
DelSiTech is collaborating routinely with an established group of high quality preclinical CROs. DelSiTech provides fast and cost-effective access to early preclinical PK/PD studies for its partners together with bioanalytical services. Also many preclinical efficacy models can be run. Please contact us for a confidential discussion on your specific needs.
Why work with us
DelSiTech offers you a one-stop shop opportunity in drug delivery up to early clinical proof-of concept.
When working with DelSiTech, you can choose from a simple, initial feasibility study up to a full development program for a new product. You can always stop the project at any stage or transfer the technology to your own site.
With the DelSiTechTMSilica Matrix you can:
- Create novel product opportunities
- Extend and manage life-cycle of existing products
- Differentiate from competitors
- Obtain additional IPR for low-IPR products
- Administer difficult-to-administer products
